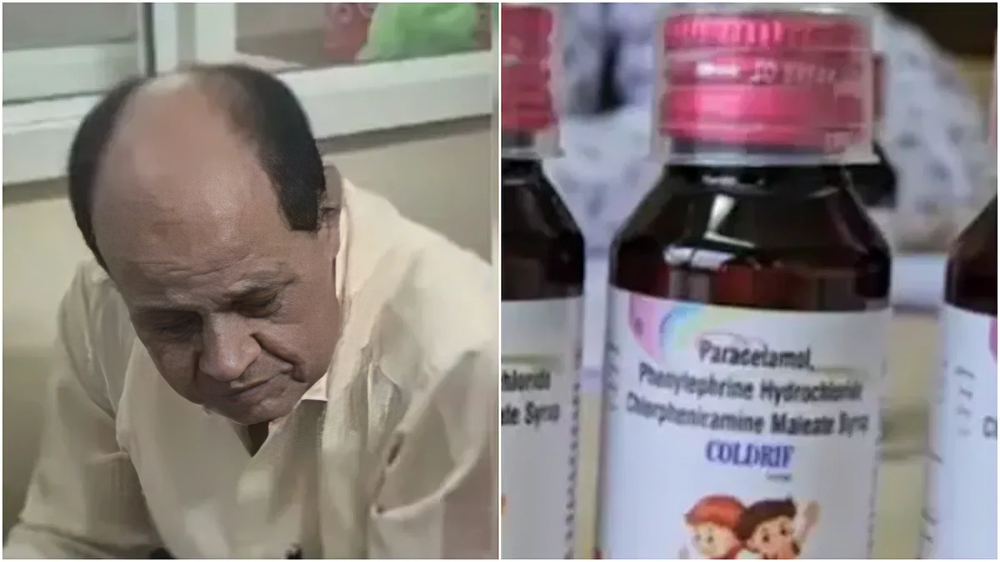
On Monday, the Tamil Nadu government cancelled the license of Sresan Pharmaceuticals. This came after Sresan Pharmaceuticals manufactured contaminated cough syrup, Coldrif, leading to the deaths of 22 children in Madhya Pradesh. The company, which is situated in the Kancheepuram district, has been directed to close the shop.
State health department directs pharma closure
The state’s health department said, “The license to manufacture has been entirely cancelled and the firm has been closed down.” The authorities also notified the Union Ministry of Health and Family Welfare and the other two states-Odisha and Puducherry, where the syrup was sold.
Owner arrested
A Special Investigation Team (SIT) from Madhya Pradesh arrested G Ranganathan, the owner of Sresan Pharma, from his Chennai residence last week. The state drug inspectors in Kancheepuram were suspended by the Tamil Nadu government for not conducting regular checks of the pharma unit since 2022.
Toxic Chemicals found
The scandal was exposed after the drug authority of Madhya Pradesh notified its Tamil Nadu counterpart on October 1 about the tainted batch. Tamil Nadu, post-notification, also made its own investigation, and it tested samples from the same batch, which showed contamination. The syrup was supposedly prepared from non-pharmacopoeial grade Propylene Glycol, which might have been contaminated with Diethylene Glycol (DEG) and Ethylene Glycol, both known nephrotoxic and toxic chemicals. Tests showed a whopping 48.6% DEG in the samples, 486 times the allowed limit.
After the detection, Tamil Nadu banned the sale of Coldrif altogether to stop further use. The state assured that government hospitals and clinics were not impacted, since all medicines are purchased through the Tamil Nadu Medical Services Corporation (TNMSC). The "stop production" order was given to Sresan Pharma on October 3, and a show-cause notice asking for an explanation of the license violation was issued to Ranganathan and analytical chemist K. Maheswari on October 7 with 10 days to respond.
The case has sparked mass outrage and fresh demands for closer monitoring of pharmaceutical production standards in India. Investigations are underway to establish the extent of negligence and contamination, with legal action imminent against all concerned.